Case Study
The BAMP Study
UNIVERSITY HOSPITAL OF SOUTH MANCHESTER
The BAMP Study is a multicentre randomised clinical trial to investigate whether Bone Anchored Maxillary Protraction (BAMP) reduces the need for orthognathic (facial) surgery.
The BAMP Study: Summary
Children with reverse bite and large lower jaws have what is termed a class III skeletal pattern. In some cases, the problem is that the upper jaw is set too far back and the treatment is to bring it forwards to correct the reverse bite.
Braces alone cannot correct this gap. In the UK patients do not usually receive corrective treatment until their general and facial growth is complete at 17-18 years of age. At this point, they may be offered jaw surgery to correct the jaw position.
Between the ages 11- 14, patients are often distressed, teased or bullied about their jaw at a time when peer opinion is particularly important to them. Bone Anchored Maxillary Protraction (BAMP) has been used in Europe for 11- 14 year old children to correct their class III jaw relationship without having to wait until they have finished growing.
BAMP consists of small metal plates that are inserted from inside the mouth into the front part of the cheekbone bone next to the upper molars on each side. Further miniplates are positioned into the bone down in the lower canine region near the front of the lower jaw. Elastics are then attached from the upper to the lower plates, via hooks, to bring the upper jaw and teeth forwards and correct the class III skeletal pattern. The mini-plates are placed and later removed as a day-case general anaesthetic procedure and the patient wears the elastics for around 6-8 months.
The aim of the BAMP trial was to compare the effectiveness of BAMP compared with no treatment. We will then be able to evaluate whether BAMP in the age group of 11 to 14 years old reduces the need for surgery in older teenage years.
What we did
The BAMP Study was the first of the live TRECA trials being run by the University of York. We had previously produced a test set of resources based on a live trial being run at Alder Hey Children’s hospital.
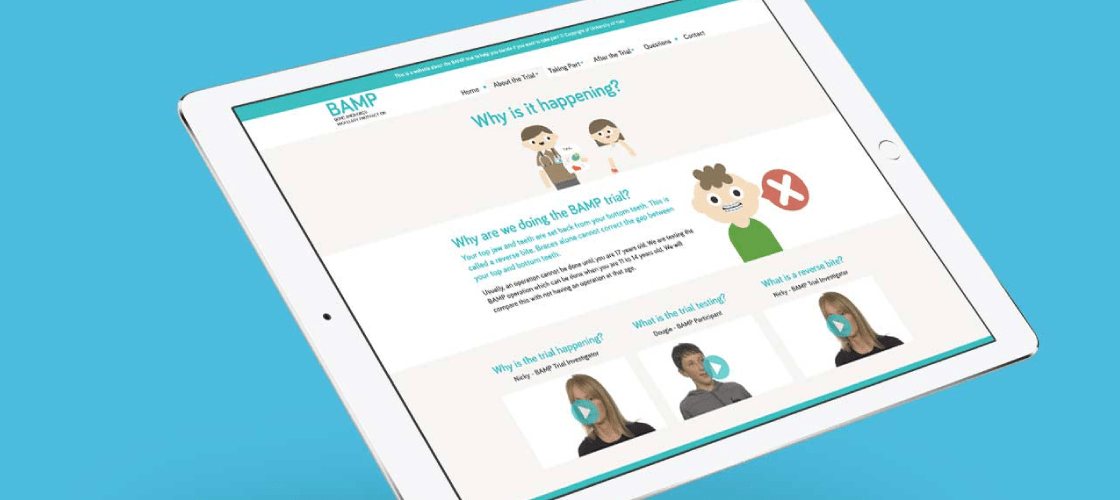
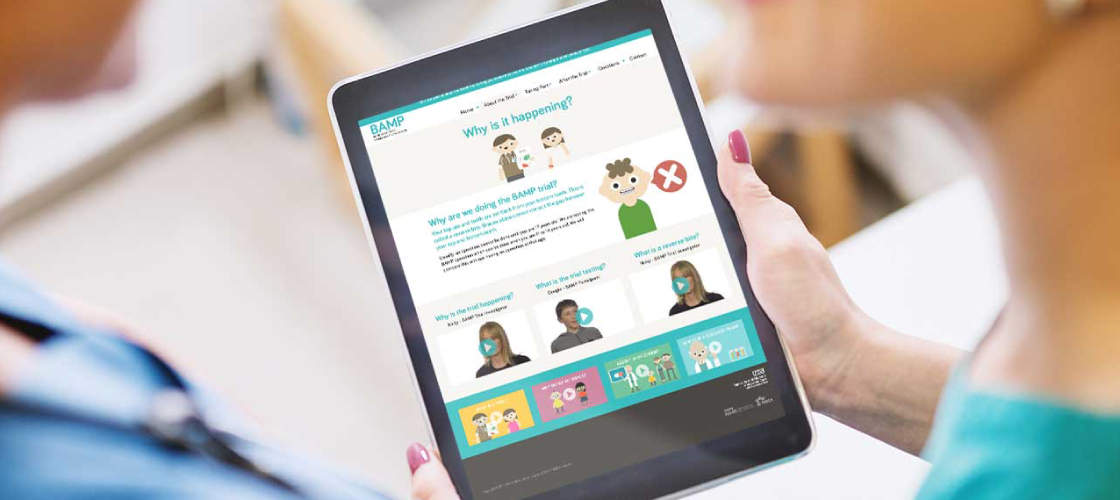
We created a two versions of the Patient Information Website to communicate the key information to the children target audience of the trial and their parents. Both versions of the website included the participant Information Animation on the home page and Talking Heads Video content. In this case the video felt like it worked particularly well because we were able to film a child who had recently had the procedure even though he was not part of the actual trial. This meant he was able to describe in practical ways what it involved.
The websites also included the set of generic Clinical Trial animations featured below.
BAMP Participant Information Animation
BAMP Participant Information Animation
This is the Participant Information Animation that we made for the home page of the BAMP Trial Patient Information Website.
BAMP Talking Head Video
BAMP Talking Head Video
This is an example of one of the Talking Heads videos that we used throughout the BAMP Patient Information Website. As well as the Principal Investigator we included talking heads from patients and parents.
BAMP Study